Examples of research projects in the Lapi Lab include:
Production of titanium-45 labeled PSMA agents
The development of new agents for Positron Emission Tomography (PET) allows investigators to monitor biological parameters in both research studies and over the course of patient treatment in the clinical setting. These imaging studies can provide important insights into the physiology of the tumor which can lead to implementation of optimized therapeutic strategies and can also be used to determine if the patient is responding to therapy.
In particular, prostate cancer, which is the second leading cancer cause of death among men in the United States, can be more accurately assessed with novel imaging techniques targeting Prostate Specific Membrane Antigen (PSMA) which is commonly overexpressed on prostate cancer cells. In current research studies, [68Ga]PSMA-11 has been widely used as an imaging agent for both local and metastatic prostate cancer. 68Ga (t1/2 = 68 min) is produced through the decay of 68Ge in the form of generators which allow for routine access to 68Ga. However, these generators are becoming very expensive ($120k) and need to be replaced about once a year. Additionally, the very short half-life of 68Ga limits the distribution of 68Ga radiopharmaceuticals. To address these problems, we propose titanium-45 (t1/2 = 3.08 h) as a impactful PET imaging radioisotope based on its promising nuclear and chemical properties, including its decay characteristics of 85% positron emission with Eβ+max = 1.04 MeV.
The goal of this project is to develop and characterize 45Ti imaging agents for targeted PSMA imaging in animal models of prostate cancer. This project will result in a new PSMA imaging agent with the potential for widespread distribution as well as pave the way for new 45Ti imaging agents.
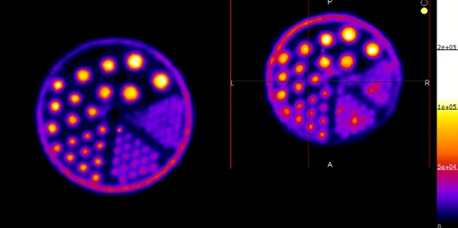
Above. PET images acquired using 18F (Left panel) 45Ti (right panel). These images show comparable resolution for 45Ti to the commonly used 18F.
Development of 55Co-labeled imaging agents
Recently, 55Co (t1/2 = 17.5 h) has garnered increased interest as a positron-emitting radionuclide due to its chemical properties and its high-abundance positron efficiency of 76%. Its half-life is suitable for the labeling of small molecules and peptides, allowing for PET-imaging up to 48 h post-injection if necessary. It is often compared with 64Cu (t1/2 = 12.7 h), which has a similar half-life but can suffer from reduction in vivo leading to undesired liver uptake. Similar in vivo behavior has not been observed with 55Co-labeled agents.
At the UAB Cyclotron Facility, 55Co is produced via the (p,α) reaction pathway using an enriched 58Ni solid target. Currently, the Lapi group has several ongoing collaborations focused on developing different 55Co-labeled radiopharmaceuticals. One of these agents is for monitoring areas of rapid bone turnover, which has implications for the detection of osteocarcinomas.
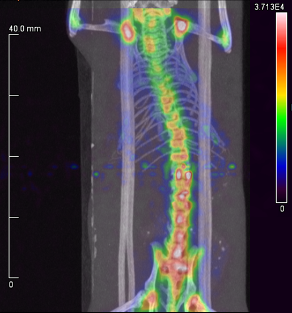
Above. A small animal PET/CT scan of a healthy mouse 4 h after injection with a 55Co-labeled phosphonate derivative. Uptake is largely limited to areas of rapid bone turnover (i.e., joints) and does not accumulate in the kidneys or liver.
Production, separation, and use of 52Mn
The novel positron-emitting isotope, 52Mn (t1/2 = 5.6 d) offers the potential for probing longer biological processes or investigating manganese-specific applications. Currently, 52Mn is being routinely produced at UAB’s Cyclotron Facility. In addition to providing 52Mn to other research institutions, the Lapi group has several projects that focus on 52Mn production and use.
One of these projects is the continual optimization of more effective and faster separation methods of radioactive Mn from Cr. This includes the development of an automated system, to reduce the absorbed dose to personnel during target processing. To further improve production of 52Mn, the cross-section of the 52Cr(p,n)52Mn reaction is being evaluated, as well as the associated 54Mn and 51Cr impurities produced when natural Cr is bombarded with protons.
Because of its half-life, 52Mn is a suitable candidate for labeling molecules with relatively long biological half-lives (e.g., monoclonal antibodies or nanoparticles). Thus, new 52Mn chelation strategies are being explored that can potentially be used to create novel imaging probes. Additionally, with the recent increase of interest in PET/MRI as well as new developments in Mn contrast agents for MRI imaging, the Lapi group is studying the characterization and quantification of 52Mn by PET/MRI, which will be useful for the development of new Mn-based MRI agents.
Development of the theranostic matched pair 44Sc & 47Sc for nuclear imaging and therapy
In nuclear medicine, a theranostic agent is one that has both therapeutic and diagnostic capabilities. The power of this combined approach has implications for enhancing and improving personalized medicine. Recently, increased development of receptor-targeted radiotherapies has highlighted the need for reliable predictions of the pharmacokinetic profiles of these agents. One of the best ways to do this is by imaging with a chemically identical, diagnostic counterpart. Therefore, the development of matched radionuclide pairs is a rapidly expanding area of research. One promising matched pair with theranostic potential is positron-emitting 44Sc (PET-imaging agent) and beta-emitting 47Sc (therapeutic agent).
In the Lapi group, production of 44Sc (t1/2 = 4.0 h) and 47Sc (t1/2 = 3.3 d) is being investigated using natural Ti targets via the 47Ti(p,α)44Sc and 50Ti(p,α)47Sc reactions. Initial work has focused on method development for the chemical separation of radioactive Sc from Ti. Future work will focus on targetry and recycling chemistry using enriched 47Ti and 50Ti. Several 44/47Sc-labeled agents are being developed.
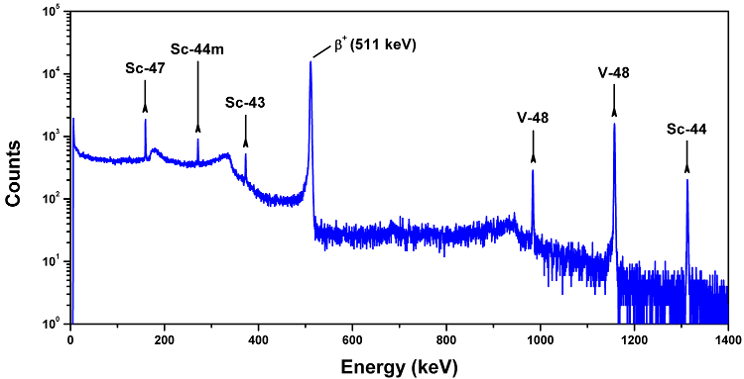
Above. A gamma-ray spectrum showing peaks attributed to several Sc radioisotopes and 48V, which were produced during a routine bombardment of a natural Ti target with 24 MeV protons.
Development and study of 18F-labeled perfluorinated alkyl substances
Perfluorinated alkyl substances (PFASs) are a family of compounds made up of polyfluorinated, carbon chain backbones with varying functional groups such as carboxylic acids, sulfonates and sulfonamines. They are used in a variety of manufacturing processes and are found in many different consumer products (e.g., non-stick cookware, flame-retardants, carpet, upholstery and fast food wrappers). These compounds have been associated with various health hazards and there is a great need to accurately assess where these compounds accumulate in the body. Unfortunately, PFASs are not easily detectable using UV spectroscopy, and so most techniques for assessing bioaccumulation are highly involved, labor intensive, and oftentimes provide contradictory results. This made the opportunity to radiolabel PFASs very exciting.
The Lapi group was interested in developing a procedure to efficiently radiolabel varying chain lengths (C4 - C8) of perfluorinated carboxylic acids with 18F (t1/2 = 109.8 min), providing a simple and straightforward way to quantitatively analyze these substances in vivo. After radiolabeling and purification, three PFASs ([18F]PFBA, [18F]PFHxA, and [18F]PFOA) were evaluated for their stability in various sera as well as their behavior in mice. Interestingly, each [18F]PFAS compound showed differing affinities for mouse, human, and rat sera, and all of the compounds displayed different biodistribution profiles within the tissues of healthy mice. To read more about these results please see our recent paper. Efforts are currently underway to create more [18F]PFAS compounds that will mimic commonly found PFASs in order to evaluate their biological behavior. Furthermore, the [18F]PFASs are being used as a way to assess more traditional purification methods.
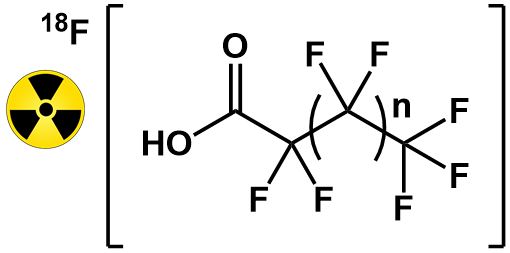
Above. The [18F]PFASs most recently developed and studied in the Lapi group. n = 1, 3, 5